The characterization of aging is of great importance for social well-being and for the devastating impact of some neurological diseases associated with aging. Functional magnetic resonance imaging (fMRI) is an investigation tool capable of capturing the deterioration of the network functionality of the human brain associated with aging and neurodegeneration. However, fMRI suffers from limitations due to the variation of physiological noise with age , which is a confounding factor that is difficult to separate from the effect of neuronal origin. This project aims to address this scientific problem, which has important industrial implications in the health sector and biomedical industry, by characterizing the physiological origin of fMRI noise in aging and in the early stages of neurodegeneration in terms of changes of vascular reactivity and of micro-motion. We will also develop appropriate mitigation methods.
The project was funded by the Lazio Regional Government under POR FESR LAZIO 2014 - 2020 A0375-2020-36648, POR A0375E0085 grant (€ 149089.39).
Minimizing the effects of noise is a major challenge in functional magnetic resonance imaging (fMRI) in order to accurately recover the signal originating from neural activity. Higher spatial resolutions are possible at ultra-high (UH) magnetic fields (7T and above) than at more typical magnitudes. Nevertheless, UH-fMRI is also more susceptible to physiological and thermal noise. The best technique for denoising UH-fMRI data is still up for debate.
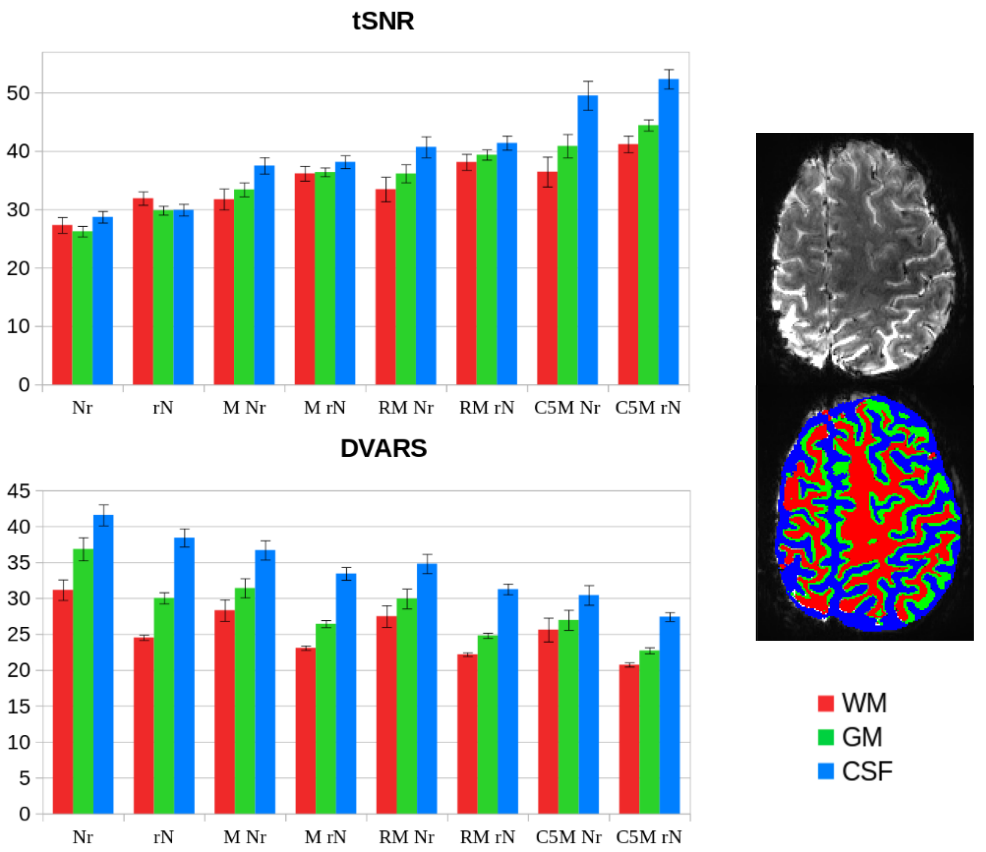
Using 7T resting-state fMRI data collected from seven healthy participants (three runs each), together with physiological (cardiac and respiratory) recordings, we evaluated the efficacy of few denoising pipelines based on recent developed algorithms (such as NORDIC [N], RETROICOR [R], and aCompCor [C5]). Each pipeline was created by permuting the order of the following analysis steps: motion correction, thermal denoising, and physiological denoising. Two quality metrics were computed to assess their efficacy: delta variation signal (DVARS) and temporal signal-to-noise ratio (tSNR). Additionally, we assessed the spatial distribution of physiological noise throughout the brain and its reproducibility across fMRI runs.
Compared to unprocessed fMRI data, all examined denoising methods enhanced the evaluated quality metrics, albeit to varying degrees, depending on the approach and the brain tissues taken into account. The figure shows the tSNR and DVARS values of the denoised datasets, averaged across all subjects and all fMRI runs, in the different tissues (white matter, WM, gray matter, GM, and cerebrospinal fluid, CSF). The analysis showed that applying thermal denoising (N) as the last step resulted in almost no improvement for all tissues. Conversely, performing N as the very first step consistently improved the quality metrics in all pipelines, as shown in figure, mainly in GM and WM. Finally, the quality ratings were enhanced by integrating physiological denoising as well, more so with aCompCor (C5MrN) than RETROICOR (RMrN). The aCompCor pipeline (C5MrN) achieved the best tSNR and DVARS values overall, with the greatest improvements for CSF, followed by GM and WM.
Regarding the spatial distribution of physiological noise, our results showed that the most affected brain tissue was the CSF, which also presented the highest level of repeatability among runs. These findings could help improve region-of-interest-based denoising algorithms (like aCompCor) by making it easier to identify the most dependable source of noise to exclude from the fMRI signal, making the neural activity detection more reliable.