 Brain activity at rest is characterized by widely distributed and spatially specific patterns of synchronized low-frequency blood-oxygenation level-dependent (BOLD) fluctuations, which correspond to physiologically relevant brain networks. This network behaviour is known to persist also during task execution, yet the details underlying task-associated modulations of within- and between-network connectivity are largely unknown.
Brain activity at rest is characterized by widely distributed and spatially specific patterns of synchronized low-frequency blood-oxygenation level-dependent (BOLD) fluctuations, which correspond to physiologically relevant brain networks. This network behaviour is known to persist also during task execution, yet the details underlying task-associated modulations of within- and between-network connectivity are largely unknown.
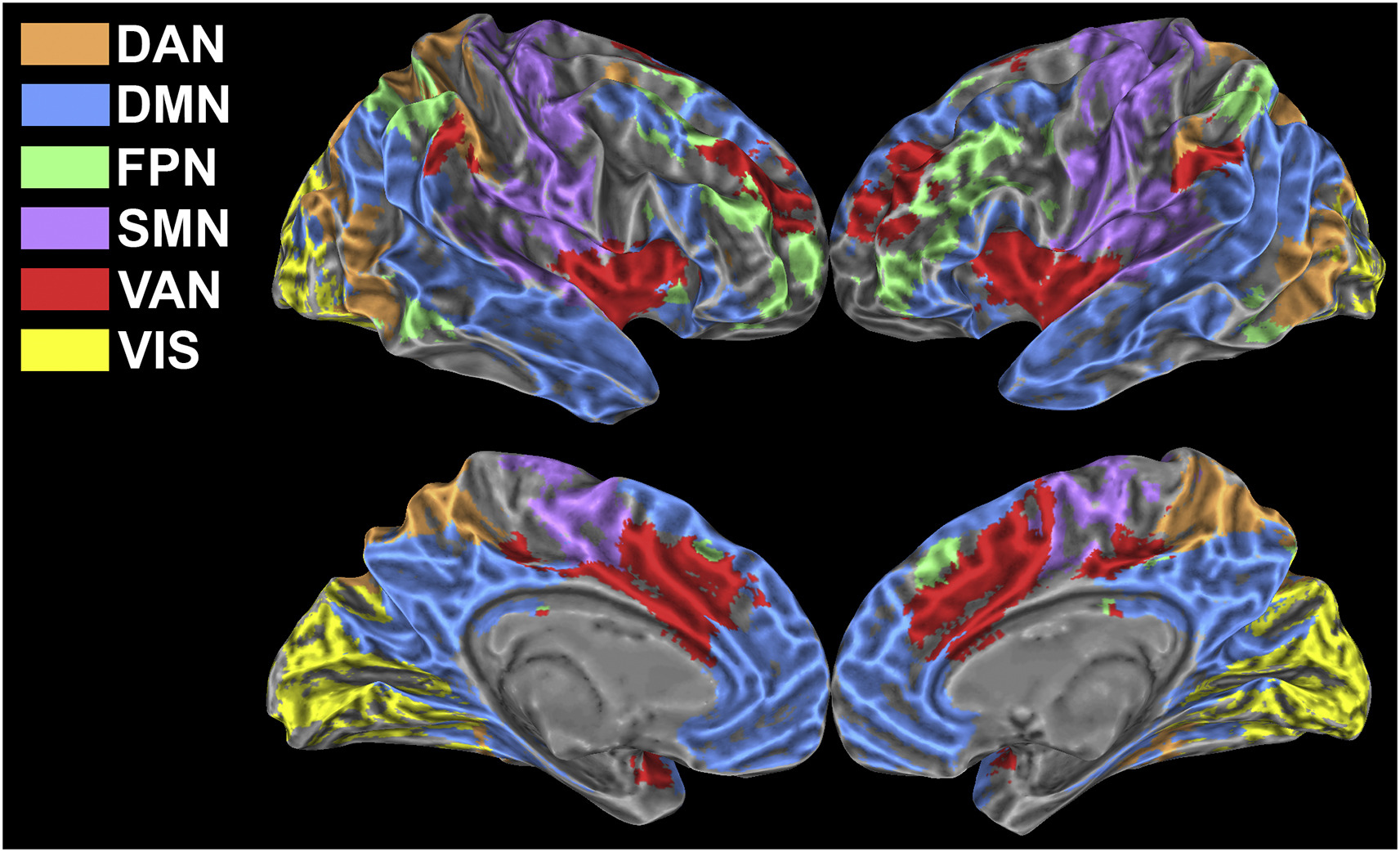
In this study we exploited a multi-parametric and multi-scale approach to investigate how low-frequency fluctuations adapt to a sustained n-back working memory task. We found that the transition from the resting state to the task state involves a behaviourally relevant and scale-invariant modulation of synchronization patterns within both task-positive and default mode networks. Specifically, decreases of connectivity within networks are accompanied by increases of connectivity between networks. In spite of large and widespread changes of connectivity strength, the overall topology of brain networks is remarkably preserved. We show that these findings are strongly influenced by connectivity at rest, suggesting that the absolute change of connectivity (i.e., disregarding the baseline) may not be the most suitable metric to study dynamic modulations of functional connectivity. Our results indicate that a task can evoke scale-invariant, distributed changes of BOLD fluctuations, further confirming that low frequency BOLD oscillations show a specialized response and are tightly bound to task-evoked activation.


